Ideal for Stomach Tissue Homogenization
Do you spend lots of time and effort homogenizing stomach tissue samples? The Bullet Blender® tissue homogenizer delivers high quality and superior yields. No other homogenizer comes close to delivering the Bullet Blender’s winning combination of top-quality performance and budget-friendly affordability. See below for a stomach tissue homogenization protocol.
Save Time, Effort and Get Superior Results with
The Bullet Blender Homogenizer
Consistent and High Yield Results
Run up to 24 samples at the same time under microprocessor-controlled conditions, ensuring experimental reproducibility and high yield. Process samples from 10mg or less up to 3.5g.No Cross Contamination
No part of the Bullet Blender ever touches the tissue – the sample tubes are kept closed during homogenization. There are no probes to clean between samples.Samples Stay Cool
The Bullet Blenders’ innovative and elegant design provides convective cooling of the samples, so they do not heat up more than several degrees. In fact, our Gold+ models hold the sample temperature to about 4ºC.Easy and Convenient to Use
Just place beads and buffer along with your tissue sample in standard tubes, load tubes directly in the Bullet Blender, select time and speed, and press start.Risk Free Purchase
Thousands of peer-reviewed journal articles attest to the consistency and quality of the Bullet Blender homogenizer. We offer a 2 year warranty, extendable to 4 years, because our Bullet Blenders are reliable and last for many years.Stomach Tissue Homogenization Protocol
Sample size |
See the Protocol |
| microcentrifuge tube model (up to 300 mg) | Small stomach samples |
| 5mL tube model (100mg - 1g) | Medium stomach samples |
What Else Can You Homogenize? Tough or Soft, No Problem!
The Bullet Blender can process a wide range of samples including organ tissue, cell culture, plant tissue, and small organisms. You can homogenize samples as tough as mouse femur or for gentle applications such as tissue dissociation or organelle isolation.

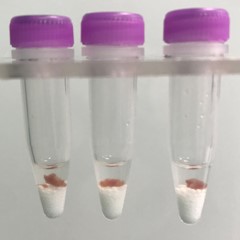
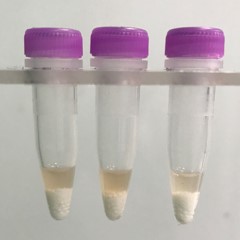
Stomach tissue pieces (on beads in upper photo) are completely homogenized into the buffer (darker in lower photo).
Want more guidance? Need a quote? Contact us:

Bullet Blender Models
Select Publications using the Bullet Blender to Homogenize Stomach Tissue
2474232
stomach
1
apa
50
date
desc
3265
https://www.nextadvance.com/wp-content/plugins/zotpress/
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22VMAN2KNB%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Rocha%20et%20al.%22%2C%22parsedDate%22%3A%222016-08%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BRocha%2C%20B.%20S.%2C%20Lundberg%2C%20J.%20O.%2C%20Radi%2C%20R.%2C%20%26amp%3B%20Laranjinha%2C%20J.%20%282016%29.%20Role%20of%20nitrite%2C%20urate%20and%20pepsin%20in%20the%20gastroprotective%20effects%20of%20saliva.%20%26lt%3Bi%26gt%3BRedox%20Biology%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B8%26lt%3B%5C%2Fi%26gt%3B%2C%20407%26%23x2013%3B414.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.redox.2016.04.002%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.redox.2016.04.002%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Role%20of%20nitrite%2C%20urate%20and%20pepsin%20in%20the%20gastroprotective%20effects%20of%20saliva%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22B%5Cu00e1rbara%20S.%22%2C%22lastName%22%3A%22Rocha%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jon%20O%22%2C%22lastName%22%3A%22Lundberg%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rafael%22%2C%22lastName%22%3A%22Radi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jo%5Cu00e3o%22%2C%22lastName%22%3A%22Laranjinha%22%7D%5D%2C%22abstractNote%22%3A%22%5Cu4469%5Cu6574%5Cu6172%5Cu7920%5Cu6e69%5Cu7472%5Cu6174%5Cu6520%5Cu6973%5Cu206e%5Cu6f77%5Cu2072%5Cu6563%5Cu6f67%5Cu6e69%5Cu7a65%5Cu6420%5Cu6173%5Cu2061%5Cu6e20%5Cu616c%5Cu7465%5Cu726e%5Cu6174%5Cu6976%5Cu6520%5Cu7375%5Cu6273%5Cu7472%5Cu6174%5Cu6520%5Cu666f%5Cu7220%5Cu6e69%5Cu7472%5Cu6963%5Cu206f%5Cu7869%5Cu6465%5Cu2028%5Cue280%5Cua24e%5Cu4f29%5Cu2070%5Cu726f%5Cu6475%5Cu6374%5Cu696f%5Cu6e20%5Cu696e%5Cu2074%5Cu6865%5Cu2067%5Cu7574%5Cu2e20%5Cu5468%5Cu6973%5Cu206e%5Cu6f76%5Cu656c%5Cu2070%5Cu6174%5Cu6877%5Cu6179%5Cu2069%5Cu6d70%5Cu6c69%5Cu6573%5Cu2074%5Cu6865%5Cu2073%5Cu6571%5Cu7565%5Cu6e74%5Cu6961%5Cu6c20%5Cu7265%5Cu6475%5Cu6374%5Cu696f%5Cu6e20%5Cu6f66%5Cu206e%5Cu6974%5Cu7261%5Cu7465%5Cu2074%5Cu6f20%5Cu6e69%5Cu7472%5Cu6974%5Cu652c%5Cu20e2%5Cu80a2%5Cu4e4f%5Cu2061%5Cu6e64%5Cu206f%5Cu7468%5Cu6572%5Cu2062%5Cu696f%5Cu6163%5Cu7469%5Cu7665%5Cu206e%5Cu6974%5Cu726f%5Cu6765%5Cu6e20%5Cu6f78%5Cu6964%5Cu6573%5Cu2062%5Cu7574%5Cu2074%5Cu6865%5Cu2070%5Cu6879%5Cu7369%5Cu6f6c%5Cu6f67%5Cu6963%5Cu616c%5Cu2072%5Cu656c%5Cu6576%5Cu616e%5Cu6365%5Cu206f%5Cu6620%5Cu7468%5Cu6573%5Cu6520%5Cu6f78%5Cu6964%5Cu616e%5Cu7473%5Cu2068%5Cu6173%5Cu2072%5Cu656d%5Cu6169%5Cu6e65%5Cu6420%5Cu656c%5Cu7573%5Cu6976%5Cu652e%5Cu2057%5Cu6520%5Cu6861%5Cu7665%5Cu2070%5Cu7265%5Cu7669%5Cu6f75%5Cu736c%5Cu7920%5Cu7368%5Cu6f77%5Cu6e20%5Cu7468%5Cu6174%5Cu2064%5Cu6965%5Cu7461%5Cu7279%5Cu206e%5Cu6974%5Cu7269%5Cu7465%5Cu2066%5Cu7565%5Cu6c73%5Cu2061%5Cu6e20%5Cu6869%5Cu7468%5Cu6572%5Cu746f%5Cu2075%5Cu6e72%5Cu6563%5Cu6f67%5Cu6e69%5Cu7a65%5Cu6420%5Cu6e69%5Cu7472%5Cu6174%5Cu696e%5Cu6720%5Cu7061%5Cu7468%5Cu7761%5Cu7920%5Cu6174%5Cu2061%5Cu6369%5Cu6469%5Cu6320%5Cu6761%5Cu7374%5Cu7269%5Cu6320%5Cu7048%5Cu2c20%5Cu7468%5Cu726f%5Cu7567%5Cu6820%5Cu7768%5Cu6963%5Cu6820%5Cu7065%5Cu7073%5Cu696e%5Cu6f67%5Cu656e%5Cu2069%5Cu7320%5Cu6e69%5Cu7472%5Cu6174%5Cu6564%5Cu2069%5Cu6e20%5Cu7468%5Cu6520%5Cu6761%5Cu7374%5Cu7269%5Cu6320%5Cu6d75%5Cu636f%5Cu7361%5Cu2c20%5Cu7969%5Cu656c%5Cu6469%5Cu6e67%5Cu2061%5Cu206c%5Cu6573%5Cu7320%5Cu6163%5Cu7469%5Cu7665%5Cu2066%5Cu6f72%5Cu6d20%5Cu6f66%5Cu2070%5Cu6570%5Cu7369%5Cu6e20%5Cu696e%5Cu2076%5Cu6974%5Cu726f%5Cu2e20%5Cu4865%5Cu7265%5Cu2c20%5Cu7765%5Cu2064%5Cu656d%5Cu6f6e%5Cu7374%5Cu7261%5Cu7465%5Cu2074%5Cu6861%5Cu7420%5Cu7065%5Cu7073%5Cu696e%5Cu2069%5Cu7320%5Cu6e69%5Cu7472%5Cu6174%5Cu6564%5Cu2069%5Cu6e20%5Cu7669%5Cu766f%5Cu2061%5Cu6e64%5Cu2065%5Cu7870%5Cu6c6f%5Cu7265%5Cu2074%5Cu6865%5Cu2066%5Cu756e%5Cu6374%5Cu696f%5Cu6e61%5Cu6c20%5Cu696d%5Cu7061%5Cu6374%5Cu206f%5Cu6620%5Cu7072%5Cu6f74%5Cu6569%5Cu6e20%5Cu6e69%5Cu7472%5Cu6174%5Cu696f%5Cu6e20%5Cu6279%5Cu206d%5Cu6561%5Cu6e73%5Cu206f%5Cu6620%5Cu7065%5Cu7074%5Cu6963%5Cu2075%5Cu6c63%5Cu6572%5Cu2064%5Cu6576%5Cu656c%5Cu6f70%5Cu6d65%5Cu6e74%5Cu2e20%5Cu5570%5Cu6f6e%5Cu2061%5Cu646d%5Cu696e%5Cu6973%5Cu7472%5Cu6174%5Cu696f%5Cu6e20%5Cu6f66%5Cu2070%5Cu656e%5Cu7461%5Cu6761%5Cu7374%5Cu7269%5Cu6e20%5Cu616e%5Cu6420%5Cu6875%5Cu6d61%5Cu6e20%5Cu6e69%5Cu7472%5Cu6974%5Cu652d%5Cu7269%5Cu6368%5Cu2073%5Cu616c%5Cu6976%5Cu6120%5Cu6f72%5Cu2073%5Cu6f64%5Cu6975%5Cu6d20%5Cu6e69%5Cu7472%5Cu6974%5Cu6520%5Cu746f%5Cu2072%5Cu6174%5Cu732c%5Cu206e%5Cu6974%5Cu7261%5Cu7465%5Cu6420%5Cu7065%5Cu7073%5Cu696e%5Cu2077%5Cu6173%5Cu2064%5Cu6574%5Cu6563%5Cu7465%5Cu6420%5Cu696e%5Cu2074%5Cu6865%5Cu2061%5Cu6e69%5Cu6d61%5Cu6c27%5Cu7320%5Cu7374%5Cu6f6d%5Cu6163%5Cu6820%5Cu6279%5Cu2069%5Cu6d6d%5Cu756e%5Cu6f70%5Cu7265%5Cu6369%5Cu7069%5Cu7461%5Cu7469%5Cu6f6e%5Cu2e20%5Cue280%5Cua24e%5Cu4f20%5Cu7761%5Cu7320%5Cu6d65%5Cu6173%5Cu7572%5Cu6564%5Cu2069%5Cu6e20%5Cu7468%5Cu6520%5Cu6761%5Cu7374%5Cu7269%5Cu6320%5Cu6865%5Cu6164%5Cu7370%5Cu6163%5Cu6520%5Cu6265%5Cu666f%5Cu7265%5Cu2061%5Cu6e64%5Cu2061%5Cu6674%5Cu6572%5Cu206e%5Cu6974%5Cu7269%5Cu7465%5Cu2069%5Cu6e73%5Cu7469%5Cu6c6c%5Cu6174%5Cu696f%5Cu6e20%5Cu6279%5Cu2063%5Cu6865%5Cu6d69%5Cu6c75%5Cu6d69%5Cu6e65%5Cu7363%5Cu656e%5Cu6365%5Cu2e20%5Cu4174%5Cu2074%5Cu6865%5Cu2065%5Cu6e64%5Cu206f%5Cu6620%5Cu6561%5Cu6368%5Cu2070%5Cu726f%5Cu6365%5Cu6475%5Cu7265%5Cu2c20%5Cu7468%5Cu6520%5Cu7374%5Cu6f6d%5Cu6163%5Cu6827%5Cu7320%5Cu6c65%5Cu7369%5Cu6f6e%5Cu732c%5Cu2072%5Cu616e%5Cu6769%5Cu6e67%5Cu2066%5Cu726f%5Cu6d20%5Cu6761%5Cu7374%5Cu7269%5Cu6320%5Cu6572%5Cu6f73%5Cu696f%5Cu6e73%5Cu2074%5Cu6f20%5Cu6861%5Cu656d%5Cu6f72%5Cu7268%5Cu6167%5Cu6963%5Cu2075%5Cu6c63%5Cu6572%5Cu732c%5Cu2077%5Cu6572%5Cu6520%5Cu7363%5Cu6f72%5Cu6564%5Cu2e20%5Cu4e69%5Cu7472%5Cu6974%5Cu6520%5Cu696e%5Cu6372%5Cu6561%5Cu7365%5Cu6420%5Cu6761%5Cu7374%5Cu7269%5Cu6320%5Cue280%5Cua24e%5Cu4f20%5Cu6279%5Cu2032%5Cu3030%5Cu2d66%5Cu6f6c%5Cu6420%5Cu2870%5Cu266c%5Cu743b%5Cu302e%5Cu3035%5Cu2920%5Cu616e%5Cu6420%5Cu6e69%5Cu7472%5Cu6174%5Cu6564%5Cu2070%5Cu6570%5Cu7369%5Cu6e20%5Cu7761%5Cu7320%5Cu6465%5Cu7465%5Cu6374%5Cu6564%5Cu2062%5Cu6f74%5Cu6820%5Cu696e%5Cu2074%5Cu6865%5Cu2067%5Cu6173%5Cu7472%5Cu6963%5Cu206a%5Cu7569%5Cu6365%5Cu2061%5Cu6e64%5Cu2074%5Cu6865%5Cu206d%5Cu7563%5Cu6f73%5Cu6120%5Cu2870%5Cu266c%5Cu743b%5Cu302e%5Cu3035%5Cu292e%5Cu2045%5Cu786f%5Cu6765%5Cu6e6f%5Cu7573%5Cu2075%5Cu7261%5Cu7465%5Cu2c20%5Cu6120%5Cu7363%5Cu6176%5Cu656e%5Cu6765%5Cu7220%5Cu6f66%5Cu206e%5Cu6974%5Cu726f%5Cu6765%5Cu6e20%5Cu6469%5Cu6f78%5Cu6964%5Cu6520%5Cu7261%5Cu6469%5Cu6361%5Cu6c2c%5Cu2062%5Cu6c75%5Cu6e74%5Cu6564%5Cu20e2%5Cu80a2%5Cu4e4f%5Cu2064%5Cu6574%5Cu6563%5Cu7469%5Cu6f6e%5Cu2061%5Cu6e64%5Cu2069%5Cu6e68%5Cu6962%5Cu6974%5Cu6564%5Cu2070%5Cu6570%5Cu7369%5Cu6e20%5Cu6e69%5Cu7472%5Cu6174%5Cu696f%5Cu6e2c%5Cu2073%5Cu7567%5Cu6765%5Cu7374%5Cu696e%5Cu6720%5Cu616e%5Cu2075%5Cu6e64%5Cu6572%5Cu6c69%5Cu6e69%5Cu6e67%5Cu2066%5Cu7265%5Cu6520%5Cu7261%5Cu6469%5Cu6361%5Cu6c2d%5Cu6465%5Cu7065%5Cu6e64%5Cu656e%5Cu7420%5Cu6d65%5Cu6368%5Cu616e%5Cu6973%5Cu6d20%5Cu666f%5Cu7220%5Cu6e69%5Cu7472%5Cu6174%5Cu696f%5Cu6e2e%5Cu2046%5Cu756e%5Cu6374%5Cu696f%5Cu6e61%5Cu6c6c%5Cu792c%5Cu2070%5Cu6570%5Cu7369%5Cu6e20%5Cu6e69%5Cu7472%5Cu6174%5Cu696f%5Cu6e20%5Cu7072%5Cu6576%5Cu656e%5Cu7465%5Cu6420%5Cu7468%5Cu6520%5Cu6465%5Cu7665%5Cu6c6f%5Cu706d%5Cu656e%5Cu7420%5Cu6f66%5Cu2067%5Cu6173%5Cu7472%5Cu6963%5Cu2075%5Cu6c63%5Cu6572%5Cu732c%5Cu2061%5Cu7320%5Cu7468%5Cu6520%5Cu6c65%5Cu7369%5Cu6f6e%5Cu7320%5Cu7765%5Cu7265%5Cu206f%5Cu6e6c%5Cu7920%5Cu6170%5Cu7061%5Cu7265%5Cu6e74%5Cu2077%5Cu6865%5Cu6e20%5Cu7065%5Cu7073%5Cu696e%5Cu206e%5Cu6974%5Cu7261%5Cu7469%5Cu6f6e%5Cu2077%5Cu6173%5Cu2069%5Cu6e68%5Cu6962%5Cu6974%5Cu6564%5Cu2062%5Cu7920%5Cu7572%5Cu6174%5Cu652e%5Cu2049%5Cu6e20%5Cu7375%5Cu6d2c%5Cu2074%5Cu6869%5Cu7320%5Cu776f%5Cu726b%5Cu2075%5Cu6e72%5Cu6176%5Cu656c%5Cu7320%5Cu6120%5Cu6e6f%5Cu7665%5Cu6c20%5Cu6469%5Cu6574%5Cu6172%5Cu792d%5Cu6465%5Cu7065%5Cu6e64%5Cu656e%5Cu7420%5Cu6e69%5Cu7472%5Cu6174%5Cu696e%5Cu6720%5Cu7061%5Cu7468%5Cu7761%5Cu7920%5Cu696e%5Cu2077%5Cu6869%5Cu6368%5Cu2070%5Cu6570%5Cu7369%5Cu6e20%5Cu6973%5Cu206e%5Cu6974%5Cu7261%5Cu7465%5Cu6420%5Cu616e%5Cu6420%5Cu696e%5Cu6163%5Cu7469%5Cu7661%5Cu7465%5Cu6420%5Cu696e%5Cu2074%5Cu6865%5Cu2073%5Cu746f%5Cu6d61%5Cu6368%5Cu2c20%5Cu7072%5Cu6576%5Cu656e%5Cu7469%5Cu6e67%5Cu2074%5Cu6865%5Cu2070%5Cu726f%5Cu6772%5Cu6573%5Cu7369%5Cu6f6e%5Cu206f%5Cu6620%5Cu6761%5Cu7374%5Cu7269%5Cu6320%5Cu756c%5Cu6365%5Cu7273%3F%22%2C%22date%22%3A%22August%202016%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.redox.2016.04.002%22%2C%22ISSN%22%3A%222213-2317%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fpii%5C%2FS2213231716300271%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222016-06-24T15%3A50%3A55Z%22%7D%7D%2C%7B%22key%22%3A%2237PBM4GC%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Chen%20et%20al.%22%2C%22parsedDate%22%3A%222016-06-09%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BChen%2C%20I.-T.%2C%20Lee%2C%20D.-Y.%2C%20Huang%2C%20Y.-T.%2C%20Kou%2C%20G.-H.%2C%20Wang%2C%20H.-C.%2C%20Chang%2C%20G.-D.%2C%20%26amp%3B%20Lo%2C%20C.-F.%20%282016%29.%20Six%20Hours%20after%20Infection%2C%20the%20Metabolic%20Changes%20Induced%20by%20WSSV%20Neutralize%20the%20Host%26%23x2019%3Bs%20Oxidative%20Stress%20Defenses.%20%26lt%3Bi%26gt%3BScientific%20Reports%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B6%26lt%3B%5C%2Fi%26gt%3B%2C%2027732.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fsrep27732%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fsrep27732%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Six%20Hours%20after%20Infection%2C%20the%20Metabolic%20Changes%20Induced%20by%20WSSV%20Neutralize%20the%20Host%5Cu2019s%20Oxidative%20Stress%20Defenses%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22I-Tung%22%2C%22lastName%22%3A%22Chen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Der-Yen%22%2C%22lastName%22%3A%22Lee%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yun-Tzu%22%2C%22lastName%22%3A%22Huang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Guang-Hsiung%22%2C%22lastName%22%3A%22Kou%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Han-Ching%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Geen-Dong%22%2C%22lastName%22%3A%22Chang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chu-Fang%22%2C%22lastName%22%3A%22Lo%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222016-6-9%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1038%5C%2Fsrep27732%22%2C%22ISSN%22%3A%222045-2322%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.nature.com%5C%2Farticles%5C%2Fsrep27732%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222016-06-24T16%3A40%3A14Z%22%7D%7D%2C%7B%22key%22%3A%22FT8RKUUB%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Park%20et%20al.%22%2C%22parsedDate%22%3A%222016%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BPark%2C%20W.%20C.%2C%20Kim%2C%20H.-R.%2C%20Kang%2C%20D.%20B.%2C%20Ryu%2C%20J.-S.%2C%20Choi%2C%20K.-H.%2C%20Lee%2C%20G.-O.%2C%20Yun%2C%20K.%20J.%2C%20Kim%2C%20K.%20Y.%2C%20Park%2C%20R.%2C%20Yoon%2C%20K.-H.%2C%20Cho%2C%20J.-H.%2C%20Lee%2C%20Y.-J.%2C%20Chae%2C%20S.-C.%2C%20Park%2C%20M.-C.%2C%20%26amp%3B%20Park%2C%20D.-S.%20%282016%29.%20Comparative%20expression%20patterns%20and%20diagnostic%20efficacies%20of%20SR%20splicing%20factors%20and%20HNRNPA1%20in%20gastric%20and%20colorectal%20cancer.%20%26lt%3Bi%26gt%3BBMC%20Cancer%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B16%26lt%3B%5C%2Fi%26gt%3B%2C%20358.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1186%5C%2Fs12885-016-2387-x%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1186%5C%2Fs12885-016-2387-x%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Comparative%20expression%20patterns%20and%20diagnostic%20efficacies%20of%20SR%20splicing%20factors%20and%20HNRNPA1%20in%20gastric%20and%20colorectal%20cancer%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Won%20Cheol%22%2C%22lastName%22%3A%22Park%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hak-Ryul%22%2C%22lastName%22%3A%22Kim%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Dong%20Baek%22%2C%22lastName%22%3A%22Kang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jae-Suk%22%2C%22lastName%22%3A%22Ryu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Keum-Ha%22%2C%22lastName%22%3A%22Choi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gyeong-Ok%22%2C%22lastName%22%3A%22Lee%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ki%20Jung%22%2C%22lastName%22%3A%22Yun%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Keun%20Young%22%2C%22lastName%22%3A%22Kim%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Raekil%22%2C%22lastName%22%3A%22Park%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kwon-Ha%22%2C%22lastName%22%3A%22Yoon%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ji-Hyun%22%2C%22lastName%22%3A%22Cho%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Young-Jin%22%2C%22lastName%22%3A%22Lee%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Soo-Cheon%22%2C%22lastName%22%3A%22Chae%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Min-Cheol%22%2C%22lastName%22%3A%22Park%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Do-Sim%22%2C%22lastName%22%3A%22Park%22%7D%5D%2C%22abstractNote%22%3A%22%5Cu5365%5Cu7269%5Cu6e65%5Cu2f61%5Cu7267%5Cu696e%5Cu696e%5Cu652d%5Cu7269%5Cu6368%5Cu2073%5Cu706c%5Cu6963%5Cu696e%5Cu6720%5Cu6661%5Cu6374%5Cu6f72%5Cu7320%5Cu2853%5Cu5253%5Cu4673%5Cu2920%5Cu616e%5Cu6420%5Cu484e%5Cu524e%5Cu5041%5Cu3120%5Cu6861%5Cu7665%5Cu206f%5Cu6e63%5Cu6f67%5Cu656e%5Cu6963%5Cu2070%5Cu726f%5Cu7065%5Cu7274%5Cu6965%5Cu732e%5Cu2048%5Cu6f77%5Cu6576%5Cu6572%5Cu2c20%5Cu7468%5Cu6569%5Cu7220%5Cu7072%5Cu6f74%5Cu656f%5Cu6d69%5Cu6320%5Cu6578%5Cu7072%5Cu6573%5Cu7369%5Cu6f6e%5Cu7320%5Cu616e%5Cu6420%5Cu7072%5Cu6163%5Cu7469%5Cu6361%5Cu6c20%5Cu7072%5Cu696f%5Cu7269%5Cu7479%5Cu2069%5Cu6e20%5Cu6761%5Cu7374%5Cu7269%5Cu6320%5Cu6361%5Cu6e63%5Cu6572%5Cu2028%5Cu4743%5Cu2920%5Cu616e%5Cu6420%5Cu636f%5Cu6c6f%5Cu7265%5Cu6374%5Cu616c%5Cu2063%5Cu616e%5Cu6365%5Cu7220%5Cu2843%5Cu5243%5Cu2920%5Cu6172%5Cu6520%5Cu6d6f%5Cu7374%5Cu6c79%5Cu2075%5Cu6e6b%5Cu6e6f%5Cu776e%5Cu2e20%5Cu546f%5Cu2061%5Cu7070%5Cu6c79%5Cu2053%5Cu4673%5Cu2069%5Cu6e20%5Cu636c%5Cu696e%5Cu6963%5Cu732c%5Cu2065%5Cu6666%5Cu6563%5Cu7469%5Cu7665%5Cu206d%5Cu6172%5Cu6b65%5Cu7220%5Cu7365%5Cu6c65%5Cu6374%5Cu696f%5Cu6e20%5Cu616e%5Cu6420%5Cu6368%5Cu6172%5Cu6163%5Cu7465%5Cu7269%5Cu7a61%5Cu7469%5Cu6f6e%5Cu206f%5Cu6620%5Cu7072%5Cu6f70%5Cu6572%5Cu7469%5Cu6573%5Cu2069%5Cu6e20%5Cu7468%5Cu6520%5Cu7461%5Cu7267%5Cu6574%5Cu206f%5Cu7267%5Cu616e%5Cu2061%5Cu7265%5Cu2065%5Cu7373%5Cu656e%5Cu7469%5Cu616c%3F%22%2C%22date%22%3A%222016%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1186%5C%2Fs12885-016-2387-x%22%2C%22ISSN%22%3A%221471-2407%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1186%5C%2Fs12885-016-2387-x%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222016-06-24T16%3A08%3A27Z%22%7D%7D%2C%7B%22key%22%3A%22EUVQTK9R%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Falendysz%20et%20al.%22%2C%22parsedDate%22%3A%222015-10-30%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BFalendysz%2C%20E.%20A.%2C%20Lopera%2C%20J.%20G.%2C%20Lorenzsonn%2C%20F.%2C%20Salzer%2C%20J.%20S.%2C%20Hutson%2C%20C.%20L.%2C%20Doty%2C%20J.%2C%20Gallardo-Romero%2C%20N.%2C%20Carroll%2C%20D.%20S.%2C%20Osorio%2C%20J.%20E.%2C%20%26amp%3B%20Rocke%2C%20T.%20E.%20%282015%29.%20Further%20Assessment%20of%20Monkeypox%20Virus%20Infection%20in%20Gambian%20Pouched%20Rats%20%28Cricetomys%20gambianus%29%20Using%20In%20Vivo%20Bioluminescent%20Imaging.%20%26lt%3Bi%26gt%3BPLOS%20Neglected%20Tropical%20Diseases%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B9%26lt%3B%5C%2Fi%26gt%3B%2810%29%2C%20e0004130.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pntd.0004130%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pntd.0004130%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Further%20Assessment%20of%20Monkeypox%20Virus%20Infection%20in%20Gambian%20Pouched%20Rats%20%28Cricetomys%20gambianus%29%20Using%20In%20Vivo%20Bioluminescent%20Imaging%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Elizabeth%20A.%22%2C%22lastName%22%3A%22Falendysz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Juan%20G.%22%2C%22lastName%22%3A%22Lopera%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Faye%22%2C%22lastName%22%3A%22Lorenzsonn%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Johanna%20S.%22%2C%22lastName%22%3A%22Salzer%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christina%20L.%22%2C%22lastName%22%3A%22Hutson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jeffrey%22%2C%22lastName%22%3A%22Doty%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nadia%22%2C%22lastName%22%3A%22Gallardo-Romero%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Darin%20S.%22%2C%22lastName%22%3A%22Carroll%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jorge%20E.%22%2C%22lastName%22%3A%22Osorio%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tonie%20E.%22%2C%22lastName%22%3A%22Rocke%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22A.%20Desiree%22%2C%22lastName%22%3A%22LaBeaud%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222015-10-30%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1371%5C%2Fjournal.pntd.0004130%22%2C%22ISSN%22%3A%221935-2735%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.plos.org%5C%2F10.1371%5C%2Fjournal.pntd.0004130%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-12-31T20%3A36%3A31Z%22%7D%7D%2C%7B%22key%22%3A%223UNSVA46%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Booth%20et%20al.%22%2C%22parsedDate%22%3A%222015-09-17%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BBooth%2C%20J.%20S.%2C%20Salerno-Goncalves%2C%20R.%2C%20Blanchard%2C%20T.%20G.%2C%20Patil%2C%20S.%20A.%2C%20Kader%2C%20H.%20A.%2C%20Safta%2C%20A.%20M.%2C%20Morningstar%2C%20L.%20M.%2C%20Czinn%2C%20S.%20J.%2C%20Greenwald%2C%20B.%20D.%2C%20%26amp%3B%20Sztein%2C%20M.%20B.%20%282015%29.%20Mucosal-Associated%20Invariant%20T%20Cells%20in%20the%20Human%20Gastric%20Mucosa%20and%20Blood%3A%20Role%20in%20Helicobacter%20pylori%20Infection.%20%26lt%3Bi%26gt%3BFrontiers%20in%20Immunology%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B6%26lt%3B%5C%2Fi%26gt%3B.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3389%5C%2Ffimmu.2015.00466%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3389%5C%2Ffimmu.2015.00466%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Mucosal-Associated%20Invariant%20T%20Cells%20in%20the%20Human%20Gastric%20Mucosa%20and%20Blood%3A%20Role%20in%20Helicobacter%20pylori%20Infection%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jayaum%20S.%22%2C%22lastName%22%3A%22Booth%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rosangela%22%2C%22lastName%22%3A%22Salerno-Goncalves%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%20G.%22%2C%22lastName%22%3A%22Blanchard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Seema%20A.%22%2C%22lastName%22%3A%22Patil%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Howard%20A.%22%2C%22lastName%22%3A%22Kader%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anca%20M.%22%2C%22lastName%22%3A%22Safta%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lindsay%20M.%22%2C%22lastName%22%3A%22Morningstar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Steven%20J.%22%2C%22lastName%22%3A%22Czinn%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bruce%20D.%22%2C%22lastName%22%3A%22Greenwald%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marcelo%20B.%22%2C%22lastName%22%3A%22Sztein%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222015-09-17%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.3389%5C%2Ffimmu.2015.00466%22%2C%22ISSN%22%3A%221664-3224%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fjournal.frontiersin.org%5C%2FArticle%5C%2F10.3389%5C%2Ffimmu.2015.00466%5C%2Fabstract%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-12-30T17%3A13%3A28Z%22%7D%7D%2C%7B%22key%22%3A%22WEERDN3P%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Nasrollahzadeh%20et%20al.%22%2C%22parsedDate%22%3A%222015-03-06%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BNasrollahzadeh%2C%20D.%2C%20Malekzadeh%2C%20R.%2C%20Ploner%2C%20A.%2C%20Shakeri%2C%20R.%2C%20Sotoudeh%2C%20M.%2C%20Fahimi%2C%20S.%2C%20Nasseri-Moghaddam%2C%20S.%2C%20Kamangar%2C%20F.%2C%20Abnet%2C%20C.%20C.%2C%20Winckler%2C%20B.%2C%20Islami%2C%20F.%2C%20Boffetta%2C%20P.%2C%20Brennan%2C%20P.%2C%20Dawsey%2C%20S.%20M.%2C%20%26amp%3B%20Ye%2C%20W.%20%282015%29.%20Variations%20of%20gastric%20corpus%20microbiota%20are%20associated%20with%20early%20esophageal%20squamous%20cell%20carcinoma%20and%20squamous%20dysplasia.%20%26lt%3Bi%26gt%3BScientific%20Reports%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B5%26lt%3B%5C%2Fi%26gt%3B%2C%208820.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fsrep08820%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fsrep08820%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Variations%20of%20gastric%20corpus%20microbiota%20are%20associated%20with%20early%20esophageal%20squamous%20cell%20carcinoma%20and%20squamous%20dysplasia%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Dariush%22%2C%22lastName%22%3A%22Nasrollahzadeh%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Reza%22%2C%22lastName%22%3A%22Malekzadeh%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alexander%22%2C%22lastName%22%3A%22Ploner%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ramin%22%2C%22lastName%22%3A%22Shakeri%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Masoud%22%2C%22lastName%22%3A%22Sotoudeh%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Saman%22%2C%22lastName%22%3A%22Fahimi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Siavosh%22%2C%22lastName%22%3A%22Nasseri-Moghaddam%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Farin%22%2C%22lastName%22%3A%22Kamangar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christian%20C.%22%2C%22lastName%22%3A%22Abnet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bj%5Cu00f6rn%22%2C%22lastName%22%3A%22Winckler%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Farhad%22%2C%22lastName%22%3A%22Islami%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Paolo%22%2C%22lastName%22%3A%22Boffetta%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Paul%22%2C%22lastName%22%3A%22Brennan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sanford%20M.%22%2C%22lastName%22%3A%22Dawsey%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Weimin%22%2C%22lastName%22%3A%22Ye%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222015-3-6%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1038%5C%2Fsrep08820%22%2C%22ISSN%22%3A%222045-2322%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.nature.com%5C%2Farticles%5C%2Fsrep08820%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-12-31T20%3A28%3A00Z%22%7D%7D%2C%7B%22key%22%3A%22QMFUBA2W%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Wang%20et%20al.%22%2C%22parsedDate%22%3A%222015%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BWang%2C%20S.-Y.%2C%20Wang%2C%20H.-Y.%2C%20Wang%2C%20T.-E.%2C%20Wang%2C%20H.-H.%2C%20Chang%2C%20W.-H.%2C%20Chu%2C%20C.-H.%2C%20Lin%2C%20S.-C.%2C%20Yeh%2C%20H.-I.%2C%20%26amp%3B%20Shih%2C%20S.-C.%20%282015%29.%20Delayed%20healing%20of%20gastric%20ulcer%20is%20associated%20with%20downregulation%20of%20connexin%2032%20in%20the%20gastric%20mucosa.%20%26lt%3Bi%26gt%3BAdvances%20in%20Digestive%20Medicine%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B2%26lt%3B%5C%2Fi%26gt%3B%282%29%2C%2067%26%23x2013%3B73.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.aidm.2015.01.004%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.aidm.2015.01.004%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Delayed%20healing%20of%20gastric%20ulcer%20is%20associated%20with%20downregulation%20of%20connexin%2032%20in%20the%20gastric%20mucosa%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shen-Yung%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Horng-Yuan%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tsang-En%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hsueh-Hsiao%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Wen-Hsiung%22%2C%22lastName%22%3A%22Chang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cheng-Hsin%22%2C%22lastName%22%3A%22Chu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shee-Chan%22%2C%22lastName%22%3A%22Lin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hung-I%22%2C%22lastName%22%3A%22Yeh%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shou-Chuan%22%2C%22lastName%22%3A%22Shih%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2206%5C%2F2015%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.aidm.2015.01.004%22%2C%22ISSN%22%3A%2223519797%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS2351979715000055%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-31T17%3A48%3A08Z%22%7D%7D%2C%7B%22key%22%3A%22ISI7UVH4%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Booth%20et%20al.%22%2C%22parsedDate%22%3A%222014-06-19%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BBooth%2C%20J.%20S.%2C%20Toapanta%2C%20F.%20R.%2C%20Salerno-Goncalves%2C%20R.%2C%20Patil%2C%20S.%2C%20Kader%2C%20H.%20A.%2C%20Safta%2C%20A.%20M.%2C%20Czinn%2C%20S.%20J.%2C%20Greenwald%2C%20B.%20D.%2C%20%26amp%3B%20Sztein%2C%20M.%20B.%20%282014%29.%20Characterization%20and%20Functional%20Properties%20of%20Gastric%20Tissue-Resident%20Memory%20T%20Cells%20from%20Children%2C%20Adults%2C%20and%20the%20Elderly.%20%26lt%3Bi%26gt%3BFrontiers%20in%20Immunology%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B5%26lt%3B%5C%2Fi%26gt%3B.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3389%5C%2Ffimmu.2014.00294%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3389%5C%2Ffimmu.2014.00294%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Characterization%20and%20Functional%20Properties%20of%20Gastric%20Tissue-Resident%20Memory%20T%20Cells%20from%20Children%2C%20Adults%2C%20and%20the%20Elderly%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jayaum%20S.%22%2C%22lastName%22%3A%22Booth%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Franklin%20R.%22%2C%22lastName%22%3A%22Toapanta%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rosangela%22%2C%22lastName%22%3A%22Salerno-Goncalves%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Seema%22%2C%22lastName%22%3A%22Patil%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Howard%20A.%22%2C%22lastName%22%3A%22Kader%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anca%20M.%22%2C%22lastName%22%3A%22Safta%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Steven%20J.%22%2C%22lastName%22%3A%22Czinn%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bruce%20D.%22%2C%22lastName%22%3A%22Greenwald%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marcelo%20B.%22%2C%22lastName%22%3A%22Sztein%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222014-06-19%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.3389%5C%2Ffimmu.2014.00294%22%2C%22ISSN%22%3A%221664-3224%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fjournal.frontiersin.org%5C%2Farticle%5C%2F10.3389%5C%2Ffimmu.2014.00294%5C%2Fabstract%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222016-01-13T16%3A53%3A28Z%22%7D%7D%2C%7B%22key%22%3A%22CG63U65K%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Melero%20et%20al.%22%2C%22parsedDate%22%3A%222014%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BMelero%2C%20M.%2C%20Garc%26%23xED%3Ba-P%26%23xE1%3Brraga%2C%20D.%2C%20Corpa%2C%20J.%2C%20Ortega%2C%20J.%2C%20Rubio-Guerri%2C%20C.%2C%20Crespo%2C%20J.%2C%20Rivera-Arroyo%2C%20B.%2C%20%26amp%3B%20S%26%23xE1%3Bnchez-Vizca%26%23xED%3Bno%2C%20J.%20%282014%29.%20First%20molecular%20detection%20and%20characterization%20of%20herpesvirus%20and%20poxvirus%20in%20a%20Pacific%20walrus%20%28Odobenus%20rosmarus%20divergens%29.%20%26lt%3Bi%26gt%3BBMC%20Veterinary%20Research%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B10%26lt%3B%5C%2Fi%26gt%3B%281%29%2C%20968.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1186%5C%2Fs12917-014-0308-2%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1186%5C%2Fs12917-014-0308-2%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22First%20molecular%20detection%20and%20characterization%20of%20herpesvirus%20and%20poxvirus%20in%20a%20Pacific%20walrus%20%28Odobenus%20rosmarus%20divergens%29%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mar%22%2C%22lastName%22%3A%22Melero%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Daniel%22%2C%22lastName%22%3A%22Garc%5Cu00eda-P%5Cu00e1rraga%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Juan%22%2C%22lastName%22%3A%22Corpa%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Joaqu%5Cu00edn%22%2C%22lastName%22%3A%22Ortega%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Consuelo%22%2C%22lastName%22%3A%22Rubio-Guerri%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jos%5Cu00e9%22%2C%22lastName%22%3A%22Crespo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bel%5Cu00e9n%22%2C%22lastName%22%3A%22Rivera-Arroyo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jos%5Cu00e9%22%2C%22lastName%22%3A%22S%5Cu00e1nchez-Vizca%5Cu00edno%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222014%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1186%5C%2Fs12917-014-0308-2%22%2C%22ISSN%22%3A%221746-6148%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.biomedcentral.com%5C%2F1746-6148%5C%2F10%5C%2F968%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222016-01-12T17%3A25%3A07Z%22%7D%7D%2C%7B%22key%22%3A%226Z38Q8R7%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Rolig%20et%20al.%22%2C%22parsedDate%22%3A%222013-05-01%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BRolig%2C%20A.%20S.%2C%20Cech%2C%20C.%2C%20Ahler%2C%20E.%2C%20Carter%2C%20J.%20E.%2C%20%26amp%3B%20Ottemann%2C%20K.%20M.%20%282013%29.%20The%20Degree%20of%20Helicobacter%20pylori-Triggered%20Inflammation%20Is%20Manipulated%20by%20Preinfection%20Host%20Microbiota.%20%26lt%3Bi%26gt%3BInfection%20and%20Immunity%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B81%26lt%3B%5C%2Fi%26gt%3B%285%29%2C%201382%26%23x2013%3B1389.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1128%5C%2FIAI.00044-13%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1128%5C%2FIAI.00044-13%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22The%20Degree%20of%20Helicobacter%20pylori-Triggered%20Inflammation%20Is%20Manipulated%20by%20Preinfection%20Host%20Microbiota%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%20S.%22%2C%22lastName%22%3A%22Rolig%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%22%2C%22lastName%22%3A%22Cech%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22E.%22%2C%22lastName%22%3A%22Ahler%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20E.%22%2C%22lastName%22%3A%22Carter%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22K.%20M.%22%2C%22lastName%22%3A%22Ottemann%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222013-05-01%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1128%5C%2FIAI.00044-13%22%2C%22ISSN%22%3A%220019-9567%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fiai.asm.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1128%5C%2FIAI.00044-13%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-20T14%3A29%3A50Z%22%7D%7D%2C%7B%22key%22%3A%22B95DPWQ8%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Rocha%20et%20al.%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BRocha%2C%20B.%20S.%2C%20Gago%2C%20B.%2C%20Barbosa%2C%20R.%20M.%2C%20Lundberg%2C%20J.%20O.%2C%20Mann%2C%20G.%20E.%2C%20Radi%2C%20R.%2C%20%26amp%3B%20Laranjinha%2C%20J.%20%282013%29.%20Pepsin%20is%20nitrated%20in%20the%20rat%20stomach%2C%20acquiring%20antiulcerogenic%20activity%3A%20A%20novel%20interaction%20between%20dietary%20nitrate%20and%20gut%20proteins.%20%26lt%3Bi%26gt%3BFree%20Radical%20Biology%20and%20Medicine%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B58%26lt%3B%5C%2Fi%26gt%3B%2C%2026%26%23x2013%3B34.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.freeradbiomed.2012.12.017%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.freeradbiomed.2012.12.017%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Pepsin%20is%20nitrated%20in%20the%20rat%20stomach%2C%20acquiring%20antiulcerogenic%20activity%3A%20A%20novel%20interaction%20between%20dietary%20nitrate%20and%20gut%20proteins%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22B%5Cu00e1rbara%20S.%22%2C%22lastName%22%3A%22Rocha%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bruno%22%2C%22lastName%22%3A%22Gago%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rui%20M.%22%2C%22lastName%22%3A%22Barbosa%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jon%20O.%22%2C%22lastName%22%3A%22Lundberg%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Giovanni%20E.%22%2C%22lastName%22%3A%22Mann%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rafael%22%2C%22lastName%22%3A%22Radi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jo%5Cu00e3o%22%2C%22lastName%22%3A%22Laranjinha%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2205%5C%2F2013%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.freeradbiomed.2012.12.017%22%2C%22ISSN%22%3A%2208915849%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS0891584912018679%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-23T14%3A46%3A39Z%22%7D%7D%2C%7B%22key%22%3A%22TC63ICA3%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Rubio-Guerri%20et%20al.%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BRubio-Guerri%2C%20C.%2C%20Melero%2C%20M.%2C%20Esper%26%23xF3%3Bn%2C%20F.%2C%20Belli%26%23xE8%3Bre%2C%20E.%2C%20Arbelo%2C%20M.%2C%20Crespo%2C%20J.%2C%20Sierra%2C%20E.%2C%20Garc%26%23xED%3Ba-P%26%23xE1%3Brraga%2C%20D.%2C%20%26amp%3B%20S%26%23xE1%3Bnchez-Vizca%26%23xED%3Bno%2C%20J.%20%282013%29.%20Unusual%20striped%20dolphin%20mass%20mortality%20episode%20related%20to%20cetacean%20morbillivirus%20in%20the%20Spanish%20Mediterranean%20sea.%20%26lt%3Bi%26gt%3BBMC%20Veterinary%20Research%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B9%26lt%3B%5C%2Fi%26gt%3B%281%29%2C%20106.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1186%5C%2F1746-6148-9-106%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1186%5C%2F1746-6148-9-106%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Unusual%20striped%20dolphin%20mass%20mortality%20episode%20related%20to%20cetacean%20morbillivirus%20in%20the%20Spanish%20Mediterranean%20sea%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Consuelo%22%2C%22lastName%22%3A%22Rubio-Guerri%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mar%22%2C%22lastName%22%3A%22Melero%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fernando%22%2C%22lastName%22%3A%22Esper%5Cu00f3n%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Edwige%22%2C%22lastName%22%3A%22Belli%5Cu00e8re%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Manuel%22%2C%22lastName%22%3A%22Arbelo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jose%22%2C%22lastName%22%3A%22Crespo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Eva%22%2C%22lastName%22%3A%22Sierra%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Daniel%22%2C%22lastName%22%3A%22Garc%5Cu00eda-P%5Cu00e1rraga%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jose%22%2C%22lastName%22%3A%22S%5Cu00e1nchez-Vizca%5Cu00edno%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222013%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1186%5C%2F1746-6148-9-106%22%2C%22ISSN%22%3A%221746-6148%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.biomedcentral.com%5C%2F1746-6148%5C%2F9%5C%2F106%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-13T16%3A20%3A42Z%22%7D%7D%2C%7B%22key%22%3A%22ZUGK3GFA%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Rolig%20et%20al.%22%2C%22parsedDate%22%3A%222012-10-01%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BRolig%2C%20A.%20S.%2C%20Shanks%2C%20J.%2C%20Carter%2C%20J.%20E.%2C%20%26amp%3B%20Ottemann%2C%20K.%20M.%20%282012%29.%20Helicobacter%20pylori%20Requires%20TlpD-Driven%20Chemotaxis%20To%20Proliferate%20in%20the%20Antrum.%20%26lt%3Bi%26gt%3BInfection%20and%20Immunity%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B80%26lt%3B%5C%2Fi%26gt%3B%2810%29%2C%203713%26%23x2013%3B3720.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1128%5C%2FIAI.00407-12%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1128%5C%2FIAI.00407-12%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Helicobacter%20pylori%20Requires%20TlpD-Driven%20Chemotaxis%20To%20Proliferate%20in%20the%20Antrum%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%20S.%22%2C%22lastName%22%3A%22Rolig%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%22%2C%22lastName%22%3A%22Shanks%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20E.%22%2C%22lastName%22%3A%22Carter%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22K.%20M.%22%2C%22lastName%22%3A%22Ottemann%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222012-10-01%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1128%5C%2FIAI.00407-12%22%2C%22ISSN%22%3A%220019-9567%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fiai.asm.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1128%5C%2FIAI.00407-12%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-20T14%3A32%3A17Z%22%7D%7D%2C%7B%22key%22%3A%22X5VSXJVM%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Sause%20et%20al.%22%2C%22parsedDate%22%3A%222012%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BSause%2C%20W.%20E.%2C%20Castillo%2C%20A.%20R.%2C%20%26amp%3B%20Ottemann%2C%20K.%20M.%20%282012%29.%20The%20Helicobacter%20pylori%20Autotransporter%20ImaA%20%28HP0289%29%20Modulates%20the%20Immune%20Response%20and%20Contributes%20to%20Host%20Colonization.%20%26lt%3Bi%26gt%3BInfection%20and%20Immunity%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B80%26lt%3B%5C%2Fi%26gt%3B%287%29%2C%202286%26%23x2013%3B2296.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1128%5C%2FIAI.00312-12%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1128%5C%2FIAI.00312-12%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22The%20Helicobacter%20pylori%20Autotransporter%20ImaA%20%28HP0289%29%20Modulates%20the%20Immune%20Response%20and%20Contributes%20to%20Host%20Colonization%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22William%20E.%22%2C%22lastName%22%3A%22Sause%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrea%20R.%22%2C%22lastName%22%3A%22Castillo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Karen%20M.%22%2C%22lastName%22%3A%22Ottemann%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22S.%20R.%22%2C%22lastName%22%3A%22Blanke%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2207%5C%2F2012%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1128%5C%2FIAI.00312-12%22%2C%22ISSN%22%3A%220019-9567%2C%201098-5522%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fiai.asm.org%5C%2Flookup%5C%2Fdoi%5C%2F10.1128%5C%2FIAI.00312-12%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-20T14%3A35%3A12Z%22%7D%7D%2C%7B%22key%22%3A%22MA9IBGWR%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Rolig%20et%20al.%22%2C%22parsedDate%22%3A%222011-12-06%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BRolig%2C%20A.%20S.%2C%20Carter%2C%20J.%20E.%2C%20%26amp%3B%20Ottemann%2C%20K.%20M.%20%282011%29.%20Bacterial%20chemotaxis%20modulates%20host%20cell%20apoptosis%20to%20establish%20a%20T-helper%20cell%2C%20type%2017%20%28Th17%29-dominant%20immune%20response%20in%20Helicobacter%20pylori%20infection.%20%26lt%3Bi%26gt%3BProceedings%20of%20the%20National%20Academy%20of%20Sciences%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B108%26lt%3B%5C%2Fi%26gt%3B%2849%29%2C%2019749%26%23x2013%3B19754.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1073%5C%2Fpnas.1104598108%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1073%5C%2Fpnas.1104598108%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Bacterial%20chemotaxis%20modulates%20host%20cell%20apoptosis%20to%20establish%20a%20T-helper%20cell%2C%20type%2017%20%28Th17%29-dominant%20immune%20response%20in%20Helicobacter%20pylori%20infection%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%20S.%22%2C%22lastName%22%3A%22Rolig%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20E.%22%2C%22lastName%22%3A%22Carter%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22K.%20M.%22%2C%22lastName%22%3A%22Ottemann%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222011-12-06%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1073%5C%2Fpnas.1104598108%22%2C%22ISSN%22%3A%220027-8424%2C%201091-6490%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.pnas.org%5C%2Fcgi%5C%2Fdoi%5C%2F10.1073%5C%2Fpnas.1104598108%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-20T14%3A34%3A09Z%22%7D%7D%2C%7B%22key%22%3A%228ADCJV4D%22%2C%22library%22%3A%7B%22id%22%3A2474232%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Lancaster%20and%20Pfeiffer%22%2C%22parsedDate%22%3A%222010-03-05%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BLancaster%2C%20K.%20Z.%2C%20%26amp%3B%20Pfeiffer%2C%20J.%20K.%20%282010%29.%20Limited%20Trafficking%20of%20a%20Neurotropic%20Virus%20Through%20Inefficient%20Retrograde%20Axonal%20Transport%20and%20the%20Type%20I%20Interferon%20Response.%20%26lt%3Bi%26gt%3BPLoS%20Pathogens%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B6%26lt%3B%5C%2Fi%26gt%3B%283%29%2C%20e1000791.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.ppat.1000791%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.ppat.1000791%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Limited%20Trafficking%20of%20a%20Neurotropic%20Virus%20Through%20Inefficient%20Retrograde%20Axonal%20Transport%20and%20the%20Type%20I%20Interferon%20Response%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Karen%20Z.%22%2C%22lastName%22%3A%22Lancaster%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julie%20K.%22%2C%22lastName%22%3A%22Pfeiffer%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Michael%22%2C%22lastName%22%3A%22Gale%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222010-3-5%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1371%5C%2Fjournal.ppat.1000791%22%2C%22ISSN%22%3A%221553-7374%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.plos.org%5C%2F10.1371%5C%2Fjournal.ppat.1000791%22%2C%22collections%22%3A%5B%22M2MNG549%22%5D%2C%22dateModified%22%3A%222015-07-20T17%3A36%3A39Z%22%7D%7D%5D%7D
Rocha, B. S., Lundberg, J. O., Radi, R., & Laranjinha, J. (2016). Role of nitrite, urate and pepsin in the gastroprotective effects of saliva. Redox Biology, 8, 407–414. https://doi.org/10.1016/j.redox.2016.04.002
Chen, I.-T., Lee, D.-Y., Huang, Y.-T., Kou, G.-H., Wang, H.-C., Chang, G.-D., & Lo, C.-F. (2016). Six Hours after Infection, the Metabolic Changes Induced by WSSV Neutralize the Host’s Oxidative Stress Defenses. Scientific Reports, 6, 27732. https://doi.org/10.1038/srep27732
Park, W. C., Kim, H.-R., Kang, D. B., Ryu, J.-S., Choi, K.-H., Lee, G.-O., Yun, K. J., Kim, K. Y., Park, R., Yoon, K.-H., Cho, J.-H., Lee, Y.-J., Chae, S.-C., Park, M.-C., & Park, D.-S. (2016). Comparative expression patterns and diagnostic efficacies of SR splicing factors and HNRNPA1 in gastric and colorectal cancer. BMC Cancer, 16, 358. https://doi.org/10.1186/s12885-016-2387-x
Falendysz, E. A., Lopera, J. G., Lorenzsonn, F., Salzer, J. S., Hutson, C. L., Doty, J., Gallardo-Romero, N., Carroll, D. S., Osorio, J. E., & Rocke, T. E. (2015). Further Assessment of Monkeypox Virus Infection in Gambian Pouched Rats (Cricetomys gambianus) Using In Vivo Bioluminescent Imaging. PLOS Neglected Tropical Diseases, 9(10), e0004130. https://doi.org/10.1371/journal.pntd.0004130
Booth, J. S., Salerno-Goncalves, R., Blanchard, T. G., Patil, S. A., Kader, H. A., Safta, A. M., Morningstar, L. M., Czinn, S. J., Greenwald, B. D., & Sztein, M. B. (2015). Mucosal-Associated Invariant T Cells in the Human Gastric Mucosa and Blood: Role in Helicobacter pylori Infection. Frontiers in Immunology, 6. https://doi.org/10.3389/fimmu.2015.00466
Nasrollahzadeh, D., Malekzadeh, R., Ploner, A., Shakeri, R., Sotoudeh, M., Fahimi, S., Nasseri-Moghaddam, S., Kamangar, F., Abnet, C. C., Winckler, B., Islami, F., Boffetta, P., Brennan, P., Dawsey, S. M., & Ye, W. (2015). Variations of gastric corpus microbiota are associated with early esophageal squamous cell carcinoma and squamous dysplasia. Scientific Reports, 5, 8820. https://doi.org/10.1038/srep08820
Wang, S.-Y., Wang, H.-Y., Wang, T.-E., Wang, H.-H., Chang, W.-H., Chu, C.-H., Lin, S.-C., Yeh, H.-I., & Shih, S.-C. (2015). Delayed healing of gastric ulcer is associated with downregulation of connexin 32 in the gastric mucosa. Advances in Digestive Medicine, 2(2), 67–73. https://doi.org/10.1016/j.aidm.2015.01.004
Booth, J. S., Toapanta, F. R., Salerno-Goncalves, R., Patil, S., Kader, H. A., Safta, A. M., Czinn, S. J., Greenwald, B. D., & Sztein, M. B. (2014). Characterization and Functional Properties of Gastric Tissue-Resident Memory T Cells from Children, Adults, and the Elderly. Frontiers in Immunology, 5. https://doi.org/10.3389/fimmu.2014.00294
Melero, M., García-Párraga, D., Corpa, J., Ortega, J., Rubio-Guerri, C., Crespo, J., Rivera-Arroyo, B., & Sánchez-Vizcaíno, J. (2014). First molecular detection and characterization of herpesvirus and poxvirus in a Pacific walrus (Odobenus rosmarus divergens). BMC Veterinary Research, 10(1), 968. https://doi.org/10.1186/s12917-014-0308-2
Rolig, A. S., Cech, C., Ahler, E., Carter, J. E., & Ottemann, K. M. (2013). The Degree of Helicobacter pylori-Triggered Inflammation Is Manipulated by Preinfection Host Microbiota. Infection and Immunity, 81(5), 1382–1389. https://doi.org/10.1128/IAI.00044-13
Rocha, B. S., Gago, B., Barbosa, R. M., Lundberg, J. O., Mann, G. E., Radi, R., & Laranjinha, J. (2013). Pepsin is nitrated in the rat stomach, acquiring antiulcerogenic activity: A novel interaction between dietary nitrate and gut proteins. Free Radical Biology and Medicine, 58, 26–34. https://doi.org/10.1016/j.freeradbiomed.2012.12.017
Rubio-Guerri, C., Melero, M., Esperón, F., Bellière, E., Arbelo, M., Crespo, J., Sierra, E., García-Párraga, D., & Sánchez-Vizcaíno, J. (2013). Unusual striped dolphin mass mortality episode related to cetacean morbillivirus in the Spanish Mediterranean sea. BMC Veterinary Research, 9(1), 106. https://doi.org/10.1186/1746-6148-9-106
Rolig, A. S., Shanks, J., Carter, J. E., & Ottemann, K. M. (2012). Helicobacter pylori Requires TlpD-Driven Chemotaxis To Proliferate in the Antrum. Infection and Immunity, 80(10), 3713–3720. https://doi.org/10.1128/IAI.00407-12
Sause, W. E., Castillo, A. R., & Ottemann, K. M. (2012). The Helicobacter pylori Autotransporter ImaA (HP0289) Modulates the Immune Response and Contributes to Host Colonization. Infection and Immunity, 80(7), 2286–2296. https://doi.org/10.1128/IAI.00312-12
Rolig, A. S., Carter, J. E., & Ottemann, K. M. (2011). Bacterial chemotaxis modulates host cell apoptosis to establish a T-helper cell, type 17 (Th17)-dominant immune response in Helicobacter pylori infection. Proceedings of the National Academy of Sciences, 108(49), 19749–19754. https://doi.org/10.1073/pnas.1104598108
Lancaster, K. Z., & Pfeiffer, J. K. (2010). Limited Trafficking of a Neurotropic Virus Through Inefficient Retrograde Axonal Transport and the Type I Interferon Response. PLoS Pathogens, 6(3), e1000791. https://doi.org/10.1371/journal.ppat.1000791






