Ideal for Disease-Causing Mosquito Homogenization
Do you spend lots of time and effort homogenizing various disease-causing mosquito species like C.melanura (transmits eastern equine encephalitis virus), A.aegypti (transmits viruses for Zika, dengue, chikungunya, and yellow fever) and Anopheles species (transmits malaria) samples? The Bullet Blender® tissue homogenizer delivers high quality and superior yields. No other homogenizer comes close to delivering the Bullet Blender’s winning combination of top-quality performance and budget-friendly affordability. See below for a mosquito homogenization protocol.
Save Time, Effort and Get Superior Results with
The Bullet Blender Homogenizer
Consistent and High Yield Results
Run up to 24 samples at the same time under microprocessor-controlled conditions, ensuring experimental reproducibility and high yield. Process samples from 10mg or less up to 3.5g.No Cross Contamination
No part of the Bullet Blender ever touches the tissue – the sample tubes are kept closed during homogenization. There are no probes to clean between samples.Samples Stay Cool
The Bullet Blenders’ innovative and elegant design provides convective cooling of the samples, so they do not heat up more than several degrees. In fact, our Gold+ models hold the sample temperature to about 4ºC.Easy and Convenient to Use
Just place beads and buffer along with your tissue sample in standard tubes, load tubes directly in the Bullet Blender, select time and speed, and press start.Risk Free Purchase
Thousands of peer-reviewed journal articles attest to the consistency and quality of the Bullet Blender homogenizer. We offer a 2 year warranty, extendable to 4 years, because our Bullet Blenders are reliable and last for many years.Mosquito Samples Homogenization Protocol
Sample size |
See the Protocol |
| microcentrifuge tube model (up to 300 mg) | Small mosquito samples |
What Else Can You Homogenize? Tough or Soft, No Problem!
The Bullet Blender can process a wide range of samples including organ tissue, cell culture, plant tissue, and small organisms. You can homogenize samples as tough as mouse femur or for gentle applications such as tissue dissociation or organelle isolation.

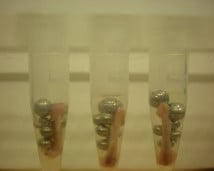

XYZ tissue pieces (on beads in upper photo) are completely homogenized into the buffer (slightly darker in lower photo).
Want more guidance? Need a quote? Contact us:







